Enrollment of patients with the correct target disease is critical to demonstrating treatment effect. However, when using only clinical diagnosis, as many as 35% of patients enrolled in Alzheimer’s disease trials have not had the target disease. This challenge increases as trials move to earlier stages of disease progression where symptoms are more subtle or not yet detectable.
ADMdx helps to assure appropriate patient inclusion by using a flexible selection of imaging modalities to align with trial needs:
Amyloid PET: | Measurement of fibrillar amyloid can rule out individuals lacking AD pathology. Our experience includes thousands of scans acquired using 11C-PiB, florbetapir, flutemetamol, florbetaben, and NAV-4694. |
Tau PET: | Neurofibrillary tangle (NFT) measurement using tau PET can identify individuals with AD pathology and who are most likely to accumulate additional NFTs. ADMdx has extensive experience in both visual reads and quantitation based on more than 2,000 MK-6240, flortaucipir, and PI-2620 tau PET images. |
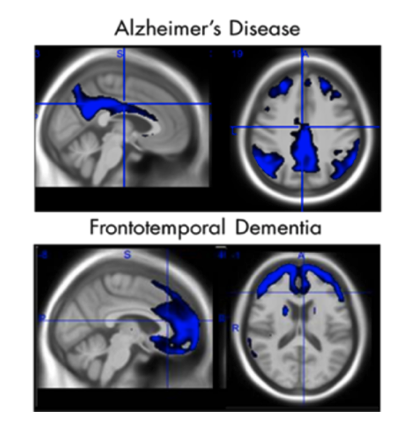
FDG PET: | AD and other dementias reduce neuronal activity in characteristic patterns. ADMdx has developed FDG PET image classifiers to evaluate the probability of AD vs. other dementias. |
MRI: | Using only an MRI scan our classifiers can help to confirm the probability of amyloid- and tau-positive AD. Since MRI is typically used for safety screens, this approach can be an efficient tool in patient inclusion. |
Plasma biomarkers: | Our recent work has evaluated the relationships between plasma biomarkers and PET imaging biomarkers of amyloid and tau, as well as the predictive power of plasma biomarkers and MRI combined. A publication on this work is forthcoming. |
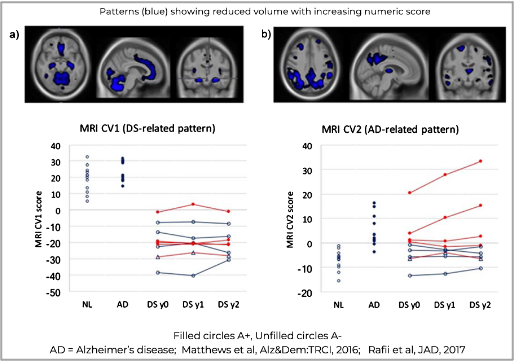
As an example, adults with Down Syndrome provide an enriched population due to the nearly 100% rate of AD pathology in this population. However, it is necessary to be able to differentiate Down Syndrome effects from AD.
Using FDG PET and MRI acquired on participants from the Down Syndrome Biomarker Initiative Study (Dr. Michael Rafii, PI), ADMdx developed image classifiers that, using either modality, dissociated the effects of progressive AD from baseline effects of Down Syndrome. Classifier scores correlated with amyloid and tau burden, as well as with cognitive endpoints. This can enable selection patients who are at target stages of disease, and to quantify treatment effects upon progressive Alzheimer’s disease neurodegeneration. See our papers in Alz&Dem and JAD.